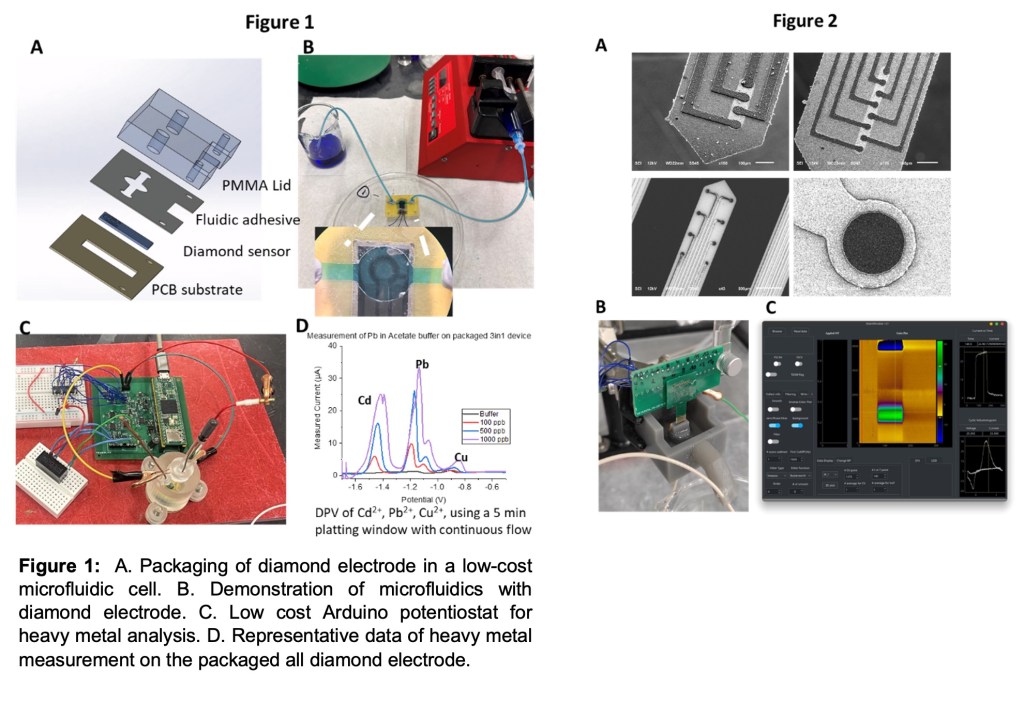
Creating robust electrochemical sensors for biological and environmental monitoring
Detecting chemical signals in biological and environmental systems is challenging due to the variability of the samples and the difficulty of controlling the conditions in which the samples are measured. To address this challenge, we have been developing boron-doped diamond (BDD) electrodes for use in harsh and complex chemical environments. BDD electrodes are p-type semiconductors grown using a chemical vapor deposition reactor. When highly doped, the material behaves as a highly conductive, metallic-like material with a non-polarizable interface. This material is advantageous as it resists both chemical and biological fouling and can be regenerated using simple cleaning methods. In our lab, we are structuring and fabricating these electrodes into all-diamond-based electrodes for neurochemical sensing, packaging them into silicon dioxide sensors for environmental sensing, and creating low-cost automation equipment for autonomous measurements. During the 10-week period, you will be paired with a mentor (an international visiting scholar) and work with them on the development and packaging strategies for these electrodes. Development includes comparing doping levels with electrochemical response, as well as packaging and creating advanced sensors for field use. You will help fabricate, collect data, analyze results, present findings to the group, and participate in the MSU undergraduate research conference.
Scientific goals:
- Packaging of diamond sensors
- Robust surface activation for long-term measurements
- Characterization of sensor response with coatings and surface modifications
Student learning goals:
- Basic understanding of electrochemical characterization of diamond material
- Packaging of a sensor for environmental usage
- Relationship of surface work, function as it relates to chemical readouts.